News and Announcements

Actinogen Medical’s First UK Site Goes Live in Global Alzheimer’s Clinical Trial
- Published June 01, 2017 12:00AM UTC
- Publisher Wholesale Investor
- Categories Company Updates
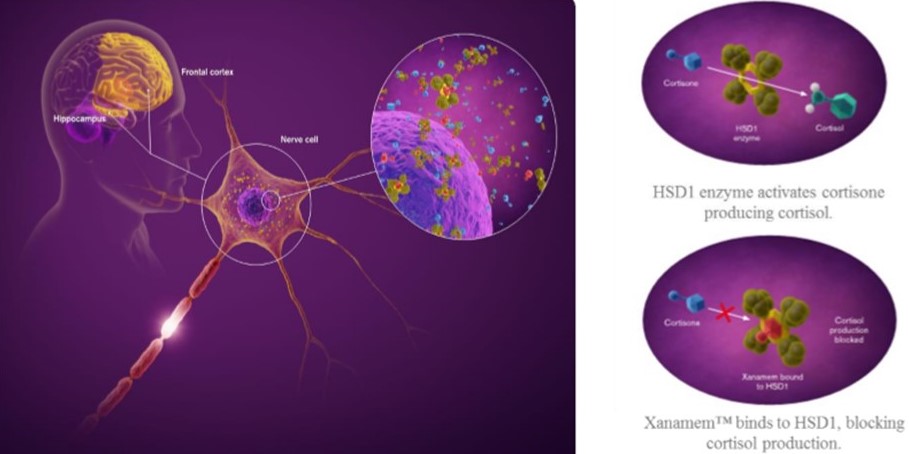
Actinogen Medical (ASX: ACW) is pleased to announce that its first UK site has opened, for patient recruitment into its clinical trial XanADu, the Company’s study of Xanamem in the treatment of Alzheimer’s disease.
KEY TAKEAWAYS:
- First UK trial site opens for patient enrolment into XanADu – Actinogen’s clinical trial evaluating Xanamem™ in Alzheimer’s disease
- UK patients can now be enrolled and treated
- This marks a significant milestone, with XanADu research sites now open in all three countries – Australia, the US and the UK
- Ten of the 20 global trial sites (50%) are now open for patient recruitment
- Opening this UK site reflects continued good momentum for the trial and follows the first patient being dosed in Australia in May 2017
Patient selection and enrolment will now commence in the UK at the University of Edinburgh Centre for Dementia Prevention, one of the most respected neuroscience institutes globally. It is the first of seven XanADu trial sites planned for the UK and follows the recent announcement of the trial’s first patient being dosed in Australia in mid-May 2017.
XanADu is a landmark study in the global search for a new and effective treatment for patients living with Alzheimer’s disease. The study is evaluating Actinogen’s new research drug Xanamem, which works by blocking the body’s excess production of the stress hormone, cortisol. Excess cortisol has been shown to be strongly associated with the development of Alzheimer’s disease, and blocking this excess cortisol production in the brain is seen as a promising approach to treating the disease.
“I am extremely pleased that patients in the UK will finally have the opportunity to receive this experimental treatment for Alzheimer’s disease” said Professor Craig Ritchie, Professor of the Psychiatry of Aging and Director of the Centre for Dementia Prevention at the University of Edinburgh. “It is also particularly pleasing to evaluate Xanamem as a potential treatment for Alzheimer’s disease, as it’s a drug discovered at Edinburgh University”, he said.
Dr Bill Ketelbey, CEO of Actinogen Medical commented: “We are very pleased with the ongoing progress of XanADu, our Alzheimer’s disease clinical trial, and with the opening of our first UK clinical trial site. It is a significant milestone for Actinogen Medical and for patients living in the UK with Alzheimer’s disease, with 50% of the trial sites now open for patient recruitment, including the first UK site.”
Company Updates
Backed By Leading Investment Groups and Family Offices







